ATOM
An atom is a particle of matter that uniquely defines a chemical element. An atom consists of a central nucleus that is surrounded by one or more negatively charged electrons. The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons.
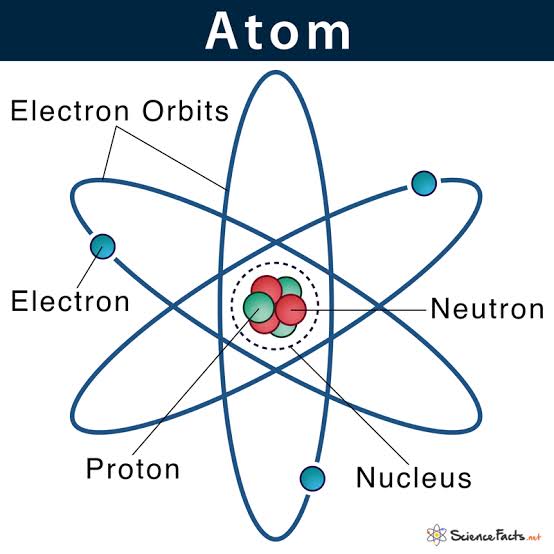
Atoms are the basic building blocks of matter.
Anything that takes up space and anything with mass is made up of atoms.
Atoms are the basic building blocks of matter. Anything that takes up space and anything with mass is made up of atoms.
ELECTRON
A neutron is electrically neutral and has a rest mass, denoted Mn, of approximately 1.675 x 10-27
The mass of a proton or neutron increases when the particle attains extreme speed, for example in a cyclotron or linear accelerator.
PROTON
A proton is positively charged. The number of protons in the nucleus of an atom is the atomic number for the chemical element. Different elements’ atomic numbers are found in the Periodic Table of Elements. For example, sodium has 11 protons, and its atomic number is
11. A proton has a rest mass, denoted My, of approximately 1.673 x 10-27 kilogram (Kg).
NEUTRON
• A neutron is electrically neutral and has a rest mass, denoted Mn, of approximately 1.675 x 10-27
The mass of a proton or neutron increases when the particle attains extreme speed, for example in a cyclotron or linear accelerator.
The structure of an atom
The total mass of an atom, including the protons, neutrons and electrons, is the atomic mass or atomic weight. The atomic mass or weight is measured in atomic mass units.
